In the past three months, the COVID-19 pandemic has turned the whole world upside-down, wreaking havoc on economies and various industrial sectors, inclusive of the life sciences industry. With the number of people infected by the pandemic surpassing two million globally, hospitals and clinics are limiting non-essential patient visits for clinical trials as traveling to the clinic/hospital for testing has become troublesome and perilous.
According to a Continuum Clinical’s recent report, approximately 80% of U.S. clinical research sites asserted that due to the impact of the global outbreak, at least one of their ongoing clinical trial studies has been put on the back burner or revoked over the past few weeks. While 81% of European clinical research sites have witnessed that most of their already-enrolled patients have discontinued participating or are less likely to participate in the ongoing trials to avoid traversing to testing sites.
| Current challenges | Short-term influence | Long-term influence | ||
|---|---|---|---|---|
| Patients dropping out of trials | Adoption of virtual trials | Rise in adoption of cloud, mobile and IoT-based wearables | ||
| Delay in trials leading to funding issues | Investments in Remote patient monitoring | Complete transformation of on-site environment to virtual one | ||
| Compromised data integrity | Real-time patient engagement measures | Large-scale adoption of remote collaboration |
Nevertheless, self-isolation, site closures, travel restrictions and other such considerations are prompting clinical research organizations (CRO) and pharmaceutical companies to incorporate remote ways of operating clinical trials with the assistance of emerging connected smart devices and technologies like Internet of Things (IoT).
“The U.S. Food and Drug Administration (FDA) urges sponsors and CROs to go virtual and conduct ‘site-less’ i.e. virtual clinical trials with remote monitoring in response to the pandemic.”
Could virtual clinical trials and remote patient monitoring take the place of traditional clinical trials? Well, the answer lies in the future. With this article, we will provide insights into how IoT-based clinical trials will reshape clinical development and revolutionize the entire trial process from “several years” to just “a couple of hours”.
Traditional clinical trials
Traditional clinical trials are essential to study the degree of efficacy and impregnability of novel drugs, but at the same time, they are expensive and tend to last a long time, sometimes years. The average expense of operating clinical studies – from developing a new drug to gaining protocol approval may range between $2 billion to $3 billion, as per a study by Johns Hopkins Bloomberg School of Public Health in 2018. Also, the entire drug development process from clinical trials to marketing can take around 12 to 18 years.
However, the cost, volume of participants and duration of a human clinical trial depends on the ongoing phase of the trial. Clinical trials are principally classified into four phases as listed below, through which researchers conduct drug experiments to gauge the side effects and strengths of a new medical treatment.
| Phase 1 | Testing of a drug on a small group of young, healthy participants to assess the possible harm and safety of the drug. | |
| Phase 2 | Testing of a drug on a large group of participants affected by the disease to test the efficacy of the drug on them. | |
| Phase 3 | Testing of a drug on a larger group of affected participants to check the effectiveness of the drug over longer periods. | |
| Phase 4 | Post-marketing surveillance trial of the drug in different groups of participants, from different countries, over a longer period to detect any long-term adverse effects of the drug (after it is approved by the FDA). |
The undeniable need to make clinical trials more patient-centric
Not only does the cost of conducting clinical studies become a challenging factor for the pharmaceutical industry, but also recruiting participants and retaining them turns out to be a major stumbling block.
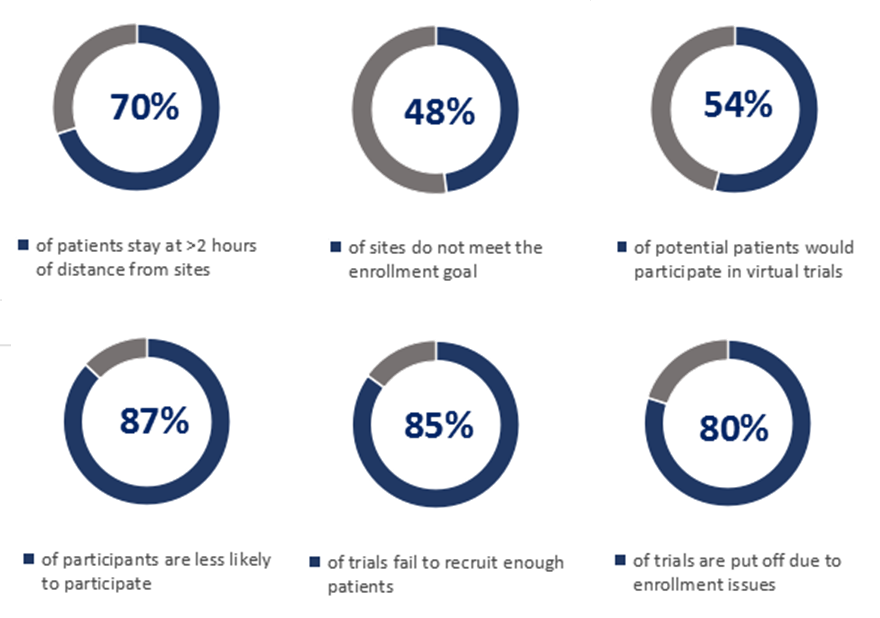
As the majority of clinical trials are conducted in large research organizations, often located in urban areas, it is difficult for the potential patients residing in rural areas or those staying at a farther distance from the sites to participate in trials. It can perpetuate the recruitment stage, potentially can even give rise to a serious challenge – it reduces the diversity of participants.
As per a U.S. research report, conducted by the Center for Information and Study on Clinical Research Participation (CISCRP) in 2018,
Hence, the FDA issued a draft guidance in June 2019, clearly stating to strengthen the diversity of participants in clinical trials. In the draft, under section “Make Trial Participation Less Burdensome for Participants”, the FDA further states to mitigate the frequency of a patients’ visit to a testing site and consider the implementation of advanced mobile tools in lieu of a site visit.
Apart from the spiraling cost of clinical trials, patient recruitment and patient diversity, other common challenges associated with conducting traditional clinical trials are:
- Manual data entry
- Inaccurate vital measurements
- Lengthy time-to-market time
- Staffing constraints
- Lack of visibility into real-world data
- Other complexity of trials
A virtual trial is a one-stop solution for “smart clinical trials”, which eases the patient’s adherence to the protocol and monitors as well as executes every step remotely. From recruiting participants to data collection, using advanced bio-wearables, smart connected devices and remote IoT-based platforms. Thanks to the Internet of Things!
How IoT streamlines clinical trials with virtual visits
The advent of the digital era and the proliferation of IoT-enabled connected devices such as bio-wearables, smartphones, in-home medical devices, technologically advanced wristwatches and telemedicine modules, has undoubtedly improved the standard of traditional clinical trials. The use of consumer-oriented IoT devices enables remote patient monitoring of a variety of medical conditions such as blood sugar, weight, heart rate, moisture, skin condition, sleep hours and much more.
To increase the flexibility of clinical trials and boost participation of mobile patients in trials, it is critical to combine technology with medical science. The state-of-the-art IoT devices not only assist CROs to improve patient-centricity by ensuring participant’s safety along with their satisfaction and convenience but also help in real-time data collection and processing. Some of the features that may seem advantageous for multiple pharmaceutical companies, sponsors and CROs are listed below:
- Works on any device, which further stimulates BYOD
- Remote patient monitoring
- Provides real-time access to data
- Trigger alerts and notifications
- Remote screening, ID verification and recruitment conducted online from any site
- Lab supplies and study medication placebo delivered to patient’s home
- Periodic self-assessments of participants via connected digital devices
As data plays a critical role in clinical monitoring, researchers must embrace advanced IoT+AI platforms and the right medical experts to integrate technology, data and analytics into risk-based monitoring (RBM) to view patient data in real-time. Centralized RBM platforms are primarily designed to:
- Enhance study quality
- Increase patient safety
- Drive new levels of monitoring
- Consolidate data on a single platform
- Add transparency to the trial status
- Take faster corrective actions
Patient-centricity is an absolute priority for research organizations, clinics and hospitals. And the only solution to achieve this outcome is to adopt and embrace a ‘Virtual Clinical Trial’. The next-gen patient will expect CROs to conduct clinical trials in a virtual environment, and therefore, we must start taking the giant steps required to meet the future needs of a diverse remote clinical trial participant.
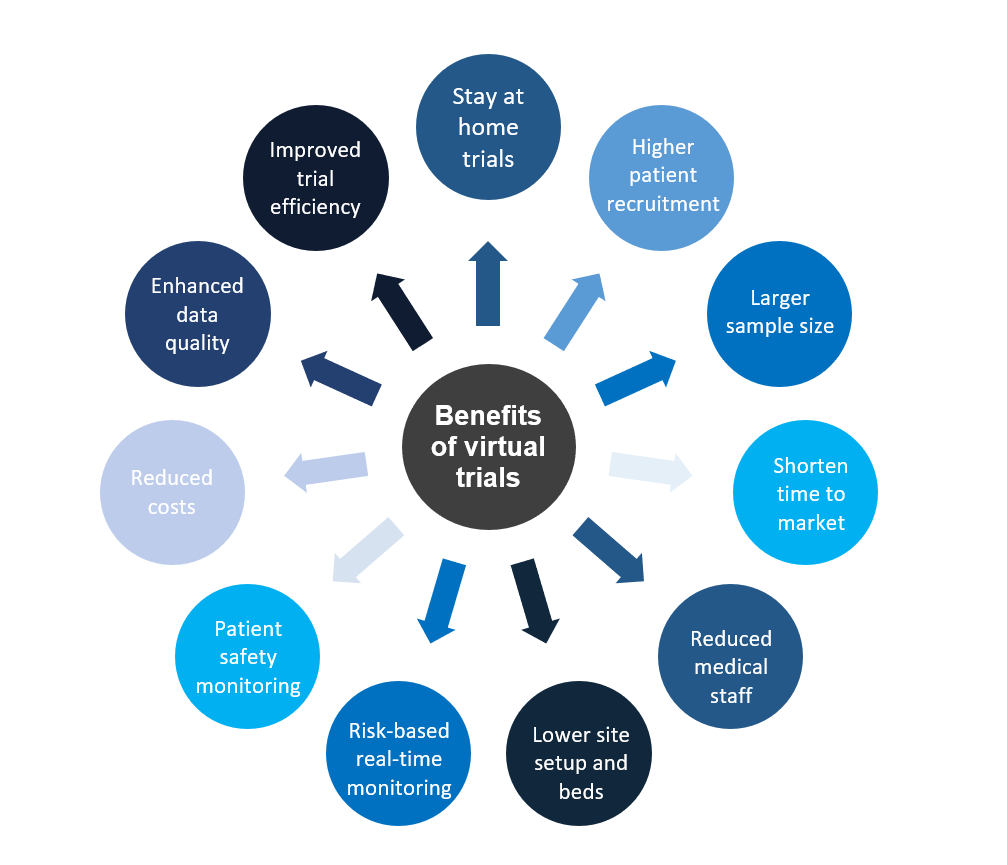
Benefits of a direct-to-patient clinical trials
How virtual are you willing to go
Technologies such as IoT hold exciting promises for the future of clinical trials. With the appropriate implementation of remote patient-facing technologies, virtual trials will bring improved treatments to patients in less time – improving their quality of life.
Virtual clinical trials are not a one-size-fits-all solution, as every trial setting (protocol) and participant population has unique clinical trial requirements, that require customization. If you want to embrace an experienced team of professionals with proven IoT-based technology for your next clinical trial, then get in touch with our team of experts.